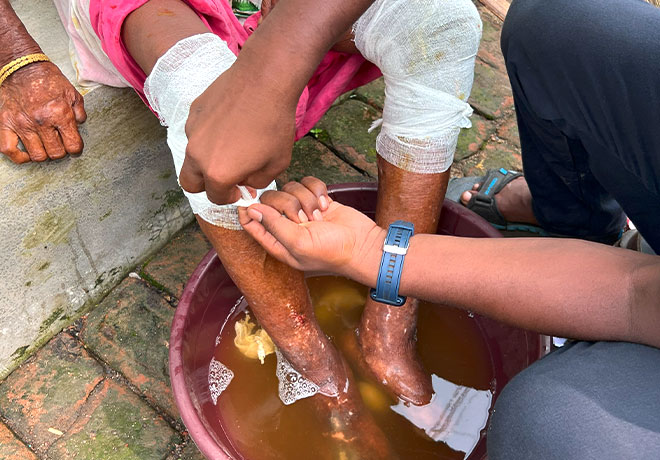
Everybody has the right to a dignified life
Bringing change at all levels
We take pride in enabling research and driving evidence-based innovation. Ongoing research on and implementation of preventive treatment provides hope that stopping leprosy transmission is coming within reach. It will take joint effort but we have the ambition to continue until No Leprosy Remains.
This is the impact YOU helped us to achieve in 2023
Your support made a significant impact in Dec 2017 – Dec 2023, driving positive change and empowering lives of person affected by leprosy through our collective efforts.
Donate for Their Happier Future!
How do you want to help today?
Your Support
All our efforts are made possible only because of your support
Tax Free Donations
Your donations are tax exempted under 80G of Income Tax Act
Secure Transactions
Your donation transactions are completely safe & secure
Three ZERO Approach
Your support made a significant impact, driving positive
change and empowering lives of persons affected by leprosy through our collective efforts.



What we do
Generating new evidence and Supporting prevention of Leprosy
We undertake innovative and ground breaking studies to inform national policy and programs to strengthening the health system in India.
We promote and support the prevention and treatment of leprosy, prevention of disabilities, social inclusion and stigma reduction of persons affected by leprosy.
SUCCESS STORIES
Leprosy Journeys: Stories of Resilience and Hope
Our organisation shares inspiring tales of persons affected by leprosy who have conquered immense challenges. We have witnessed their remarkable journeys towards self-improvement and newly acquired skills. Seeing them radiate happiness and confidence fills us with immense pride. With your support, we're committed to achieve our mission of bringing hope and positive changes to more lives of persons affected.
Stories & Updates
Articles daily updated
Your smallest contribution makes a big difference
All our efforts are made possible only because of your support